
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
![]() November 06, 2023
November 06, 2023

Abstract: Enzyme is a kind of biocatalyst with high efficiency, specificity and mild reaction conditions. Its process has the characteristics of energy saving and low environmental pollution, so it has been widely used in the textile industry. The basic characteristics of the enzyme preparation, the kinetic properties of the enzyme, the determination method of the enzyme activity, the requirements of the textile processing conditions for the stability of the enzyme preparation, and the principle of compounding the enzyme preparation are described in the article. Cellulase, protease and starch are introduced. The basic principles and production processes of enzymes, hydrolases and oxidoreductases in the printing and dyeing industry, as well as the latest developments in textile biochemical treatment technology. Key words: dyeing and finishing industry; enzyme; application
1 Basic characteristics of enzyme preparations There are many kinds of creatures on the earth. But no matter how diverse the creatures are, the continuation of their lives is inseparable from a miraculous substance, the enzyme. Almost all life activities, from animals to plants, from towering trees to microbes, require enzymes to participate. The use of enzymes can be traced back thousands of years, but the nature of enzymes was not recognized until the early 20th century, which promoted the application of biological enzymes from life sciences and food engineering to other fields, especially industrial fields. Industrial technology based on the application of enzyme preparations is considered to be a “green” process and one of the most promising technologies.

1.1 Characteristics of the enzyme To understand what an enzyme is, you must understand the following description of the enzyme. Enzymes are a special catalyst [1, 2]. A catalyst is a substance that changes the reaction rate without changing the nature of the reaction, the direction of the reaction, and the equilibrium point of the reaction, and does not change itself after the reaction is completed. For example, protein hydrolysis to amino acids, starch hydrolysis to glucose, in terms of thermodynamic properties, these reactions can be carried out spontaneously, even to the extent of complete hydrolysis, but in the absence of a catalyst, the reaction proceeds very slowly, while a small amount Acids, bases and enzymes can all catalyze these reactions and accelerate the reaction.
Compared with inorganic or organic catalysts, enzymes have the following characteristics:
(1) High catalytic efficiency In the comparable case, compared with other non-bioenzyme catalysts, the catalytic efficiency of the enzyme is as high as 107~1012 times, and some enzymes can even accelerate the reaction rate up to 1014 times.
This high catalytic efficiency of the enzyme is due to the ability of the enzyme to significantly reduce the transition state energy. For a chemical reaction, a compound that is unstable in energy to both the substrate (reactant) and the product is usually formed first. This unstable intermediate structure is called a transition state. Figure 1 is a schematic diagram of the energy relationship of three reaction transition states.
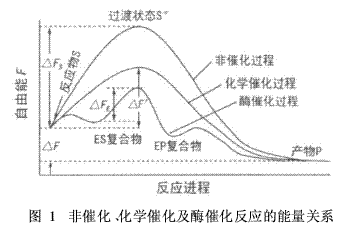
In Figure 1, ΔF is the difference between the free energy between the substrate and the product, which is the final free energy change of the reaction; ΔFS and ΔF' represent the freedom between the transition state and the substrate during the non-catalytic and chemical catalytic reactions, respectively. The difference in energy; ΔFE is the difference between the free energy between the transition state and the enzyme and substrate complex during the enzyme-catalyzed reaction. They represent the respective reaction energy barriers, the minimum energy (activation free energy) that must be possessed before the substrate is converted to the product.
Generally, for one reaction, there is a relationship of ΔFS > ΔF' ΔFE, that is, the enzymatic reaction greatly reduces the activation energy. For example, the decomposition reaction of hydrogen peroxide, in the absence of a catalyst, the activation energy is 75. 31 kJ / mo; l with palladium as a catalyst, the activation energy is 48. 99 kJ / mo; l with the enzyme as a catalyst, the activation energy is only 8. 37 kJ/mol. According to the Arrhenius equation, the reaction rate K and the reaction activation energy E have the following relationships:

Where: R———the gas constant;
A———frequency factor;
T————Absolute temperature.
Thus, the smaller the activation energy E, the faster the reaction rate. The catalyzed reaction rate of the hydrogen peroxide decomposition is 6.78×1010~3. 51×1011, for the catalase, at a temperature of 30 to 50 ° C (the optimum temperature of the catalase).倍倍。 The palladium catalyzed reaction of 6. 06 × 106 ~ 1. 14 × 107 times.
It can be seen that the activation energy of the enzyme-catalyzed reaction is significantly reduced, which is the key to the extremely high efficiency of the enzymatic reaction. The mechanism has many explanations, such as: proximity and orientation effects, substrate deformation, acid-base catalytic reaction, nucleophilic and electrophilic catalytic reactions, and the influence of the reaction microenvironment. For details, please refer to the relevant literature.
(2) High degree of specificity The specificity of an enzyme refers to the property that an enzyme can only catalyze one or a type of structurally similar substrate for a certain type of reaction. The specificity of the catalytic reaction is one of the most important properties of the enzyme, and it is the main difference between the enzyme and other non-enzymatic catalysts.
The specificity of the enzyme can be described by a variety of models, the most famous and image of which is the lock and key model, as shown in Figure 2(a), presented by Fisher in 1894. This model indicates that the molecular structure of the enzyme is fixed and indeformable in shape, which means that if the conformation of the enzyme molecule changes slightly, the relationship between the enzyme and the substrate is naturally destroyed, and the catalytic reaction cannot be performed. This is inconsistent with more and more experimental facts.
In 1958, Koshland proposed the theory of induced fit [Fig. 2(b) and 2(c)]. In 1963, Monod et al. proposed an allosteric model. These models indicate that the active site of the enzyme is flexible and can alter or enhance the activity of the enzyme even by chemical modification of the enzyme. Despite this, the enzyme lock and key model is still used to describe the highly specific properties of enzymes and substrates because of its simplicity and simplicity.

(a) Lock and key model (I) The active site of the enzyme does not match the substrate; (II) The active site of the enzyme matches the height of the substrate to catalyze the chemical reaction; (III) The active site of the enzyme matches the substrate. Low, can not efficiently catalyze chemical reactions.
(b) Induction fit model (effect) In this model, the substrate can induce the deformation of the active site of the enzyme, and the active site is matched with the substrate structure to complete the chemical reaction.
(c) The active site of the allosteric model enzyme can also deform the substrate to match the structure of the active site of the enzyme to complete the chemical reaction.
The degree of specificity of different enzymes is different, there are the following [1, 2]:
1 chemical bond specificity. The catalysis of such enzymes requires only the same chemical bonds, and is an enzyme with a low degree of specificity, such as peptidase, phosphoenzyme and esterase, which can act on many substrates. Biomolecule degradation, especially for hydrolases in the textile industry, is mostly such low specificity enzymes because relatively low specificity is more economical for degradation. Low specificity enzymes are rare in synthetases.
2 group specificity. This is a moderate degree of specificity. Most enzymes are absolutely or almost exclusively specific. They only catalyze a substrate for rapid reaction. For example, urease only catalyzes the hydrolysis of urea to carbon dioxide and water, or catalyzes very similar analogs at very low rates.
3 stereoisomerism specificity. When the substrate and the formed product of the enzyme contain asymmetric carbon atoms, the enzyme can only act on one isomer. Stereoisomer specificity is a prominent feature of enzyme-catalyzed reactions.
4 reaction specificity. An enzyme can only catalyze one of many reactions that a compound can perform thermodynamically.
The degree of specificity of the enzyme-catalyzed reaction is also related to the individual of the enzyme. The specificity of the enzyme is conducive to cost saving, improve the purity of the product, and bring convenience to industrial production. For example, amylase is used in the production of glucose to reduce water vapor by 30%, ash by 50% and by-products by 90%. The successful listing of industrialized amylase has completely replaced the acid glucose production with enzymatic methods and achieved a leap in glucose production.
(3) The reaction conditions are mild enzymes from the organism, so the general enzyme-catalyzed reactions are non-extreme conditions, except for a variety of enzymes, can be carried out under normal temperature and pressure conditions, which is conducive to production control, and can save energy and reduce equipment. cost. In addition, the enzyme-catalyzed reaction is carried out under weak acid, weak alkali or neutral conditions, which has little environmental pollution, light corrosion to equipment, and high production safety.
The activity of the enzyme is also regulated by some compounds. Some enzymes, such as chymotrypsin, do not require any cofactors when performing viability, but most enzymes require the presence of non-protein components, cofactors, to behave. Cofactors can be broadly divided into two broad categories, namely organic cofactors and metal ions. About one-third of the existing enzymes require the presence of metal ions to maintain activity. One of them has one or several metal ions in the molecular structure of the enzyme. These enzymes are called metalloenzymes; It is only active when a metal ion or a metal ligand is added. This enzyme is called a metal activating enzyme.
1.2 Active site of the enzyme The active site of the enzyme consists of a few amino acid side chain groups in the enzyme molecule. The active site typically appears as a deep "groove" or "pit" on the surface of the enzyme molecule to accommodate the catalytic site of the substrate. Some special enzymes may also be a microtubule structure, such as cellulose synthase. Figure 3 is a schematic diagram of the active structure of the enzyme "pit". When the carbonyl side of the peptide bond is an alanine residue, since the alanine side group is a methyl group, it cannot penetrate deep into the bottom of the enzyme "pit" and thus cannot be hydrolyzed. The peptide bond; conversely, for the lysine residue, since the lysine has a long side group containing an amino group, it can penetrate deep into the bottom of the "pit" to produce a complexing action at the active site of the enzyme, thereby generating hydrolysis and exhibiting enzyme activity. Specificity.

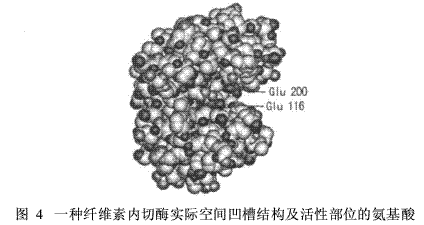
Figure 4 shows the spatial structure of a endonuclease. The active site has a groove structure and is composed of glutamic acid (Glu) at positions 116 and 200 in the primary structure.
In terms of function, several amino acid side groups of the active site can be further divided into a binding site and a catalytic site of the substrate [4]. The binding site of the substrate is the site that specifically binds to the substrate, and is therefore also called a specific or specificity determining site. The catalytic site is directly involved in the catalysis, and the sensitive bonds of the substrate are cleaved or new bonds are formed at this site, and a product is formed. The distinction between the binding site and the catalytic site of the substrate is not absolute, and some groups have the dual function of binding the substrate and catalyzing the substrate reaction. From the perspective of a well-defined enzyme, a single functional enzyme of a peptide chain has only one active site.
The group constituting the catalytic site of the enzyme is provided by a side group of an amino acid such as a hydroxyl group of serine, a thiol group of cysteine, an imidazolyl group of histidine, a carboxyl group of aspartic acid and glutamic acid, and an ε-F of lysine. Amino group, etc. The catalytically active site usually consists of 2 to 3 such amino acids [2].
Different types of enzymes have significant differences in the deep concave shape of the active site. In the case of hydrolases, the endonuclease is an elongated groove, such as a lysozyme groove that is just sized to hold six monosaccharides of the polysaccharide substrate; and the exonuclease appears as a hydrophobic pocket, such as hydroxycarboxypeptidase A. The hydrophobic pocket consists of Zn2+, His-69, His-196, Glu-72, Arg-145, Tyr-248, Glu-270, Tyr-198, Phe-279, Arg-71 and other groups (His is a group) Amino acid, Glu is glutamic acid, Phe is phenylalanine, Arg is arginine, and Tyr is tyrosine. The substrate must be combined with the active site in the correct manner to be hydrolyzed smoothly. The size of the active site of the enzyme is often a function of the size of the substrate. For macromolecular substrates, such as starch, protein and nucleic acid molecules, there may be many interaction sites between them and the enzyme. Even so, the vast majority of the enzyme peptide chain is still not directly in contact with the substrate, but these inactive site peptide chains play a role in maintaining the conformation of the active site, that is, maintaining all the groups at the optimal position of the active site [3].
Since the specificity of the enzyme depends on the side groups of several key amino acid residues in the active site, the pH state of the solution also affects the dissociation state of the side groups of these amino acid residues, thereby affecting the activity of the enzyme, and thus the enzyme has the most Good vitality pH conditions. At the same time, since the enzyme is a protein, its stability decreases with increasing temperature, and the rate of deactivation of the enzyme increases. On the other hand, according to the thermodynamic principle, the temperature rise increases the catalytic efficiency of the enzyme, and thus the enzyme has a Relatively optimal kinetic temperature. This kinetic temperature is actually a function of the time of action of the enzyme.
1.3 Classification and naming of enzymes [1, 2]
There are more than 3,000 known enzymes. In order to accurately identify an enzyme and avoid confusion and misunderstanding, it is required to have an accurate name and classification for each enzyme in the fields of enzymology research and enzyme engineering.
According to the decision of the Enzyme Commission (E. C.), the basic basis for distinguishing enzyme specificity is the reaction they catalyze. According to this, the catalytic reactions of known enzymes are divided into six categories:

Under each category, it can be divided into several sub-categories according to the type of reaction. Among them, hydrolyzing enzymes, oxidoreductases and synthetases are mainly applied and studied in the textile industry.
The International Committee of Enzymes has recommended a system nomenclature for enzymes. Usually each enzyme has a system name and a recommended common name (habit name). The type of catalytic reaction of each enzyme can be determined by the system name. It is generally composed of an enzyme-catalyzed substrate name plus a large class name of the enzyme, such as a protease and a proteolytic enzyme, the former being the common name and the latter being the system name. In the case of a double substrate reaction, both substrates are listed and separated by a colon. English enzyme names are suffixed with "ase".
In addition, each enzyme has a four-digit number (label). The first three digits of the number indicate the major classes, subclasses, and sub-categories, and the enzyme type and catalytic properties can be judged according to the first three digits; the fourth digit indicates the sequence number of the enzyme in the sub-subcategory. Based on these four numbers, specific enzymes can be determined. For example, the trypsin number is EC 3. 4. 21. 4. For the different enzymes that catalyze the same reaction, there are the same first three digits, only different in the fourth digit.
The Enzyme Commission also recommends that when publishing a paper, the enzyme should be written with its code, system name, and source when it is first mentioned, and then described by system name or custom name.
1.4 Production of enzymes 1. 4. 1 The main production methods of enzymes [1, 2]
Enzyme production methods can be divided into extraction, fermentation and chemical synthesis.
The extraction method is the earliest enzyme production method and is still in use. For example, trypsin, pancreatic amylase, pancreatic lipase , and a mixture of these enzymes, trypsin, are extracted from the animal's pancreas; papain is extracted from papaya. The extraction method is simple and convenient, but the enzyme-containing tissue is affected by the climate and geographical environment, and the product contains a lot of impurities, and it is difficult to separate and purify.
Fermentation is the main method of enzyme production since the 1950s. The method utilizes cells, mainly microbial cell life activities, to obtain the enzymes that people need. Currently, enzymes for industrial applications are mainly derived from microorganisms. This is because there are many types of microorganisms, and almost all enzymes can be found from microorganisms. Moreover, microorganisms are easy to culture, and as long as simple equipment and general raw material medium can be rapidly propagated, a large amount of enzyme is obtained.
At present, the chemical synthesis production method of enzymes is also studied. Since the first chemical synthesis in the United States in 1969 to obtain a 124 amino acid ribonuclease, through the efforts of many scientists, peptide synthesis can now be used for chemical synthesis of enzymes. However, since the various amino acids required for the synthesis are extremely high in purity, costly, and can only synthesize enzymes of known chemical structure, the chemical synthesis method is greatly limited, and it is still in the experimental stage.
1. 4. 2 The focus of production and development of industrial enzyme preparations (1) Compound processing of industrial enzyme preparations [2]
The compound processing of enzyme preparations has become an important part of the production of commercial enzyme preparations. As an industrial enzyme preparation, the conditions of enzyme action have changed greatly and have their own characteristics. Different textile enterprises have different requirements for textile enzyme preparations due to different conditions of production products, processing objects and equipment. In fact, the commercial enzyme preparations provided by the world's leading professional enzyme preparation manufacturers contain various additives to maintain the stability of the enzyme preparation and form a series of products to meet different processing requirements. In addition, in addition to directly supplying enzyme preparations for textile production, these companies also provide high-concentration enzyme preparations for textile auxiliaries and some large enterprises. In order to meet specific process conditions, companies often need to compound after purchasing enzyme products.
(2) Production of genetically modified enzyme preparations [4]
The key to the production of enzyme preparations by fermentation is the preservation of the species. One of the core technologies of a professional enzyme manufacturer is how to preserve the strain for a long time, and the type of the strain will not change for a long time:
1 Cryopreservation Normally it can be stored for two years at -5 to -20 °C. To save longer, the temperature must be controlled between -50 and -80 °C. In addition, it can be stored for a long time under liquid nitrogen conditions. Liquid nitrogen storage is considered to be the best method for long-term preservation of strains.
2 Freeze-drying method Freeze-drying is a method in which frozen cells are dehydrated by depressurization to remove water. Most strains can be stored for 10 years using this method.
3 Subculture The strain was transplanted to fresh agar by irregular, cultured at a suitable growth temperature, and then the cultured strain was stored in a refrigerator at 5 °C. This method is convenient and economical, but it is not suitable for long-term storage.
At present, the main work of enzyme preparation production is to improve the enzyme production bacteria, so that the strain can increase the enzyme production during the fermentation process, reduce or remove the hybrid enzymes and other metabolites, and reduce the fermentation cost. Therefore, the genetic modification and recombination of the production bacteria has become the focus of the current economical and efficient production of enzyme preparations. The traditional method is to screen the mutant strains under a strong genetic mutagenesis environment. This method is also known as genetic mutation, and its history dates back to the study of Thom and Steinberg in the 1930s. This method was successfully developed in the production of penicillin in the 1940s, and it has been rapidly developed. Common genetic mutagenesis environments are ultraviolet, X-ray, gamma ray radiation, chemical agents and space environments. The species that survived in this environment were classified and observed to improve the production of the enzyme preparation. In the 1950s and 1970s, this method was used for specific enzyme production, and its enzyme production performance was improved by 3 to 6 times, which greatly promoted the development of the enzyme preparation industry.
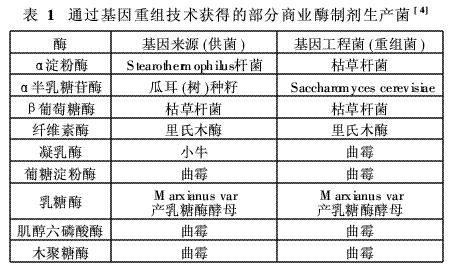
The production of enzyme preparations made a major leap in the development of genetic engineering and recombinant DNA (rDNA) technology in the 1970s. Using these techniques, the specific gene code of a specific enzyme is isolated, purified, and emphasized in the corresponding strains, so that the yield and purity of the enzyme preparation can be greatly improved. Escherichia coli is the first strain to carry out genetic recombination research. With the advancement of technology and the great application value of genetic recombination in commerce and industry, in the 1980s, people with commercial application value, such as Aspergillus (such as Aspergillus) Aspergillus ni-ger), Trichoderma, and Bacillus subtilis, etc., perform large-scale gene cloning. The first food-grade enzyme preparation produced by genetic recombination (strain) technology was alpha-amylase, and CPC International submitted a production license application to the Food and Drug Administration (FDA). Subsequently, genetic recombination technology was gradually accepted by most enzyme preparation companies and became the main means of enzyme production and development. Table 1 shows the currently commercially available genetically engineered modified enzyme preparation products, in which amylase, cellulase and xylanase are enzyme preparations which can be used in the textile industry, which will be described in detail later. The strain is the core technology of the enzyme preparation manufacturer and is strictly kept secret. Even the enzyme preparations produced by the same strain may vary greatly in their performance.
Genetic recombination technology has many advantages for the production and development of enzyme preparations, mainly in [4, 5]:
1 The enzyme preparation produced by the genetically modified bacteria can be much more pure than the original bacteria. In the food industry, pure enzyme preparations can better improve the performance of food processing, such as the need to add amylase and pentosanase in bread making. Currently, the two enzymes produced by genetic recombination technology are completely free of protease activity. The toughness of the flour is better maintained because no additional hydrolysis is applied to the gluten in the flour. The purity of the enzyme is also of particular importance in the processing of the textile industry.
2 The yield of the enzyme preparation produced by the genetically modified bacteria can be greatly increased, which also means that the production cost is lowered.
3 The production of specific enzyme preparations that can be commercialized by genetic recombination technology is theoretically feasible. The best example is the production of chymosin. The chymosin was first discovered in the stomach of calves and can now be produced by microbial fermentation because the gene fragment of the bovine was successfully expressed in Aspergillus and Marxianus var Lactase -producing yeast (MaxirenTM).
The 4 gene expression system is also called a "plug-in" system, in which a gene fragment can be selectively inserted into a target strain to improve the performance of the enzyme and is economical. This means that different types of enzymes can be produced in the same enzyme-producing system of the same species, and their commercial application value is obvious. Despite this, the use of genetically modified enzymes (foods) in the food sector is currently cautious, and its rapid development in the non-food sector, such as textile, paper and pulp production. There is no doubt that genetic recombination technology is the development direction of enzyme production.
Gene mutation and gene recombination technology have become an effective means for producing textile enzyme preparations with excellent performance, stable viability and wide-area performance. For example, the currently produced desizing amylase can be used at a high temperature of 110 °C. Genetic modification of new enzyme preparations is currently the focus of enzyme production and is also the development direction.
(3) Development and application of immobilized enzymes Although the application of enzymes is more and more extensive, some shortcomings of enzymes are also noted during the use of enzymes. Specifically in:
1 The stability of the enzyme is poor. The enzyme is easily denatured and deactivated by external factors such as temperature, pH and inorganic ions.
2 Common enzymes react with the substrate in an aqueous solution. In the reaction system, the enzyme is mixed with the substrate and the product, and after the reaction is finished, even if the enzyme still has high vigor, it is difficult to recycle. This single-use enzyme method is not only costly but also difficult to continuously produce.
3 After the enzyme catalyzes the reaction, the enzyme is mixed with impurities and products, which brings certain difficulties for further separation and purification.
In response to these shortcomings of enzymes, people have been seeking ways to improve for a long time. Immobilization technology is the most effective result of long-term research.
After the enzyme or the enzyme-containing cells are made into immobilized or immobilized cells, the properties of the enzyme will change due to the influence of the carrier and the immobilization method, such as the optimum temperature, the optimum pH and the substrate specificity. Etc. But the most important thing is that the stability of the immobilized enzyme is significantly better than that of the free enzyme, mainly in:
1 The stability of heat is improved and can withstand higher temperatures;
2 The storage stability is good, and it can be stored for a long time under certain conditions;
3 is highly resistant to proteases and is not easily hydrolyzed by proteases;
4 The tolerance to the denaturant is improved, and the viability of the protein, such as urea, organic solvent and guanidine hydrochloride, can still maintain high vigor.
There are many methods for immobilization of enzymes, but mainly adsorption, entrapment (embedding), binding, cross-linking, covalent bonding, ion exchange, and heat treatment. After the enzyme is immobilized, the specificity of the enzyme will change, which is caused by the steric hindrance of the carrier. After the enzyme is immobilized on the carrier, the macromolecule is difficult to access the enzyme molecule, and the catalytic efficiency is greatly reduced. The immobilized enzyme has no effect on the macromolecular product or has a low efficiency, while the substrate with a small molecular mass is sterically hindered due to steric hindrance. The effect is small or unaffected, and there is no significant difference between catalytic efficiency and free enzyme.
The immobilization of enzymes has many potential applications in textiles, such as immobilization of enzymes on fabrics, which makes the fabrics have biological antibacterial effects [6]; while oxidoreductases are immobilized, they can be used as biological oxygen bleaching wastewater purification and sewage ( Recycled materials for wastewater treatment have good application prospects.
1.5 Conclusion Enzyme is a kind of protein, and it is a special catalyst with high efficiency, specificity and mild reaction conditions. This property determines the way it is used differently than ordinary chemicals. Enzymes have an optimal range of active pH values and an optimal range of activity temperatures associated with the time of action. As a biocatalyst, enzymes maintain their viability and stability is the key to industrial applications. In recent years, with the development of bioengineering, the application of genetic recombination and gene modification technology, a group of enzymes with stable vitality, wide pH value and wide temperature range, which can meet the requirements of textile industry production and application have been effectively developed. The root cause of rapid application in the textile industry.
References: slightly
The above is the Application of biological enzymes in textile industry we have listed for you. You can submit the following form to obtain more industry information we provide for you.
You can visit our website or contact us, and we will provide the latest consultation and solutions
Send Inquiry
Most Popular
lastest New
Send Inquiry

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.